SILICON LMA Gastro
SILICON LMA Gastro Specification
- Function
- Airway management during procedures
- Shelf Life
- 5 years (unopened)
- Instruments Type
- Airway device
- Accuracy
- Clinically approved
- Usage Type
- Disposable or Reusable (as per variant)
- Storage Instructions
- Store in cool and dry place, away from sunlight
- Features
- Dual-channel design, Gastric access, Soft flexible cuff, Easy insertion, Reusable, Latex free, Sterilizable
- Equipment Type
- Supraglottic Airway Device
- Material
- Silicone
- Condition
- New
- Technology
- Medical Grade Polymer
- Portable
- Yes
- Wall Mounted
- No
- Real-Time Operation
- Yes
- Noise Level
- Silent
- Operating Type
- Manual
- Use
- Gastroenterology, Anaesthesia, Airway Management
- Dimension (L*W*H)
- Varies (Depending on Size)
- Weight
- Lightweight
- Color
- Transparent, Blue piloted cuff
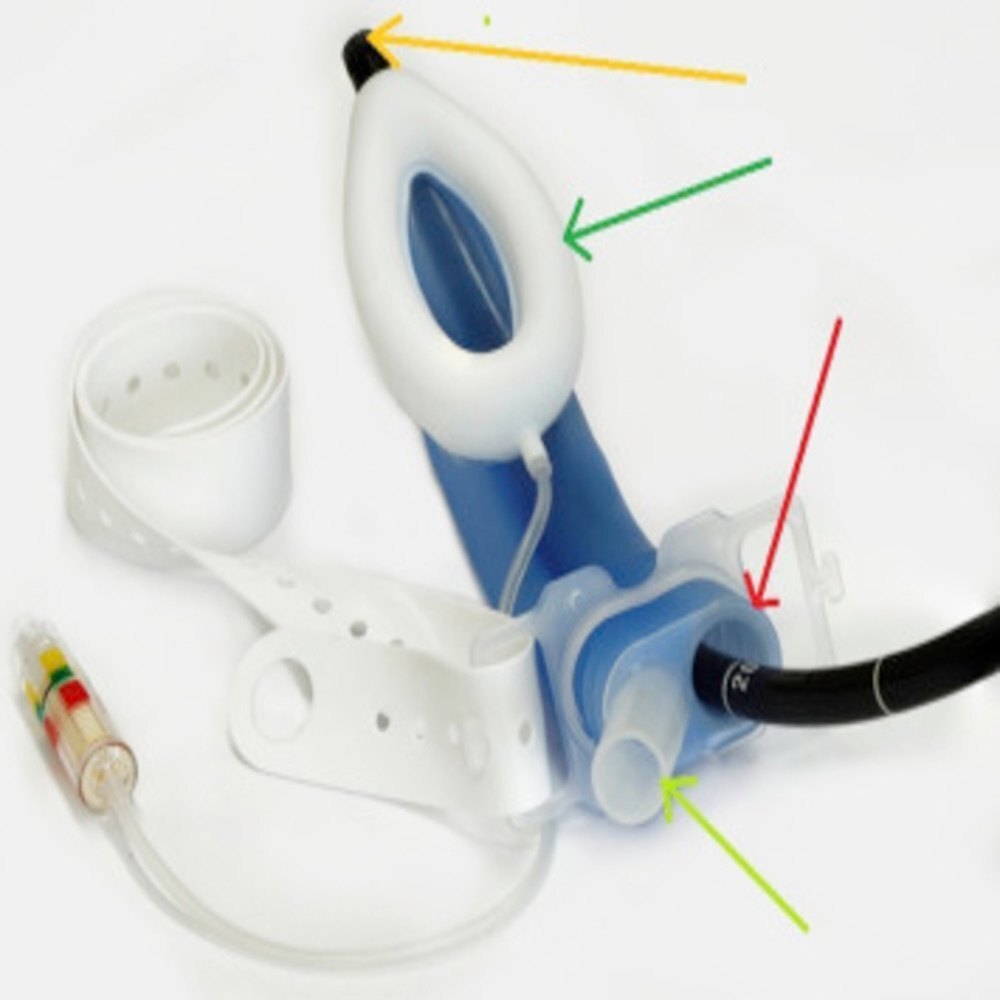
About SILICON LMA Gastro
Proactive airway management for endoscopy procedures Separate gastric and airway access Hypoxemia due to respiratory depression or airway obstruction is a known risk associated with endoscopic procedures, with studies showing that hypoxemia can occur in 1150% of cases.1-3 The LMA Gastro Airway with Cuff Pilot Technology from Teleflex is the first laryngeal mask specifically designed to enable clinicians to proactively manage their patients airways while facilitating direct endoscopic access via the integrated endoscope channel. With the airway in place, clinicians can monitor end-tidal CO2 for patient safety. Designed to support patient safety The single-use LMA Gastro Airway is designed for patient comfort with a silicone cuff that is soft and flexible, and conforms to the patient anatomy to create an effective oropharyngeal seal.4,5 Silicone cuffs have been shown to reduce risk of sore throat6 and achieve higher seal pressures4 compared with PVC cuffs. Clinician Inspires confidence by supporting end-tidal CO2 monitoring and direct endoscopic access Patient Silicone cuff and integrated cuff pressure monitoring for patient comfort Institution Designed to help reduce airway-related complications during endoscopy procedures Integrated Cuff Pilot Technology Cuff Pilot Technology is an integrated, cuff pressure indicator that constantly monitors cuff pressure, detecting changes at a glance resulting from fluctuations in temperature, nitrous oxide levels, and movements within the airway. Cuff Pilot Technology was developed to support clinicians in avoiding the known risks of cuff hyperinflation, which include sore throat, dysphagia, an increased risk of aspiration due to leakage around the cuff, and hypoglossal, lingual or recurrent laryngeal nerve palsiesDual-Channel Design for Efficient Gastroenterology Care
The SILICON LMA Gastro integrates both an airway and gastric channel, allowing for simultaneous airway protection and gastric tube insertion. This innovation enhances patient safety by preventing aspiration while facilitating procedures like endoscopy or anaesthesia. Its transparent construction and flexible blue piloted cuff make insertion intuitive and verification of placement simple.
Versatility for All Patient Populations
Available in both adult and pediatric sizes, the SILICON LMA Gastro offers tailored care for diverse patient groups. Its compatibility with standard nasogastric tubes (up to 18 Fr) adds to its clinical flexibility, making it a preferred choice for various hospital and ambulatory settings. Clinically approved with a five-year shelf life, it supports consistent, safe airway management.
Sterile, Portable, and Reliable
Each SILICON LMA Gastro device is provided sterile and ready to use, ensuring immediate applicability. The device is lightweight, portable, and can be either reusable or disposable depending on the selected variant. Carefully engineered from a high-quality medical polymer, it meets the highest standards in both performance and patient safety.
FAQs of SILICON LMA Gastro:
Q: How is the SILICON LMA Gastro inserted and what lubrication is required?
A: The SILICON LMA Gastro should be inserted manually with care, using a water-based lubricant to aid placement. The flexible and soft silicone design facilitates a smooth insertion process for both adult and pediatric patients.Q: What are the benefits of the dual-channel design in this airway device?
A: The dual-channel configuration enables simultaneous airway management and gastric tube insertion, reducing the risk of aspiration and improving safety during gastroenterology and anaesthesia procedures.Q: When should the SILICON LMA Gastro be used?
A: This device is designed for airway management during gastroenterology procedures, anaesthesia, or any situation where both airway protection and gastric access are required, particularly in elective or emergency settings.Q: Where should the SILICON LMA Gastro be stored before use?
A: For optimal longevity and sterility, store the device in a cool, dry place away from direct sunlight. Unopened, it has a shelf life of five years.Q: What makes this product suitable for both adult and pediatric patients?
A: The SILICON LMA Gastro is available in multiple sizes to accommodate different anatomies, ensuring secure placement and ease of use for patients of all ages.Q: Is the device reusable, and how should it be sterilized if so?
A: Depending on the variant chosen, the SILICON LMA Gastro can be either reusable or disposable. Reusable units should be sterilized according to standard medical protocols before each use.Q: What are the key features that enhance the devices performance in clinical practice?
A: Its latex-free, clinically approved silicone structure, transparent body, dual-channel configuration, and compatibility with NG tubes up to 18 Fr all contribute to accurate placement, patient safety, and effective airway management.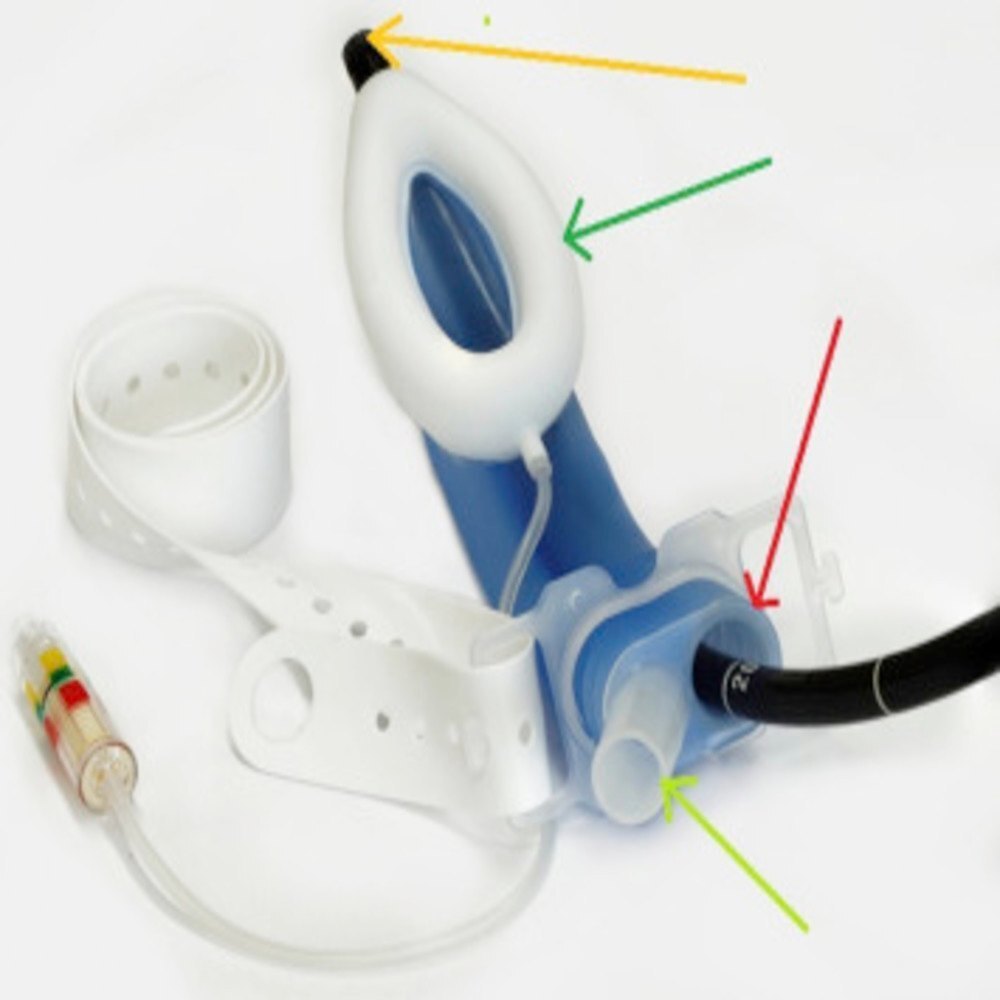
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Intubation Devices And Accessories Category
KELLY RETRACTOR
Price 1200 INR / Piece
Minimum Order Quantity : 10 Pieces
Condition : New
Material : Other, Stainless Steel
Color : Silver
Use : Used for holding back tissue or organs during surgery
Laryseal Clear LMA
Price 850 INR / Piece
Minimum Order Quantity : 10 Pieces
Condition : New
Material : Other, Medical Grade Silicone
Color : Transparent
Use : Airway Management during Anesthesia
MODERMA FLEX DRAINABLE POUCH/BAG 26801
Price 1650.00 INR / Piece
Minimum Order Quantity : 10 Pieces
Condition : New
Material : Plastics
Color : Translucent/Clear
Use : Ostomy Care
DES JARDIN FORCEPS
Price 1900 INR / Piece
Minimum Order Quantity : 10 Pieces
Condition : New
Material : Stainless Steel
Color : Silver





 Send Inquiry
Send Inquiry Send SMS
Send SMS
